Gee, Brain, what do you want to do tonight?
— 11th November 2009
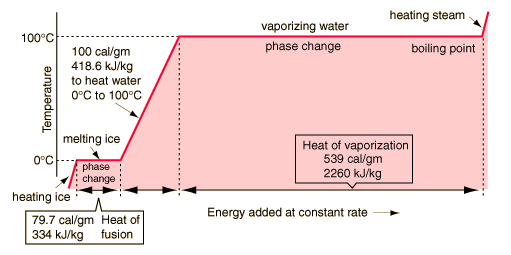
Look at this graph. It’s a graph from a website but it looks very similar to a graph in a science experiment I did at school.
It basically tracks the temperature of water as you heat it. All the way from ice through it’s melting point (0°C) up to and through it’s boiling point (100°C). Ha, like you didn't know that.
What I want to discuss is the point at the top of the graph there where the water turns into steam, where it boils. I remember the lesson where we drew this graph. I don't really know why although I think perhaps that it’s because of that point at the top of the graph. What we found was that as you heat the water consistently the temperature increases, also consistently, up to a point (100°C) and at that point the temperature stops increasing. The reason it stops is because although you're applying heat to the water the energy of that heat is no longer being used to raise the temperature but to convert the liquid into steam. They call it a phase transition and it requires energy. You can see in the graph there that it takes quite a lot of energy because it takes quite a while for the temperature to start increasing again.
Right so where are we? We're heating water. Good because kettles is what I really want to talk about. With your kettle you heat water and this happens using electricity which is forced through a wire which is resistant to having electricity forced through it, and because of the way of the world works this resistance causes the wire to heat up (think of it as angry at the imposition) and this heats your water.
That’s not the only thing kettles do. Most kettles nowadays perform the neat trick of turning themselves off again after the water is boiled. This saves the element from getting too angry (furious?) and stops the whole process before all the water has turned to steam so you’ve got some left to drink.
I wasn't sure how kettles did this so I looked it up. It turns out they use a thing called a bimetallic strip. This is something else I learned about in school (oh, the british education system). Basically this is a small strip made of two types of metal, one on one side and the other on the other. The trick is that one metal is more susceptible to heating up than the other meaning it expands more causing the strip to curl as one side gets longer faster than the other.
This is very handy when it comes to turning things off when they get hot. Basically you set up your strip in such a way as it trips a switch when it gets hot and you’ve got a self-turn-offing kettle. Woot!
Can you see the problem yet? Right here's the thing. Most kettles are set up in such a way as their bimetallic strip is heated by steam. This steam is created as the water boils and so it a pretty good indicator that the water is boiling, and is therefore a pretty good indicator that the kettle should now be turned off. But wait! Why are we using the kettle in the first place? To heat water. Our goal here is hot water not steam. Think back to the graph. After the water reached 100C the energy we're adding is being used only to create steam. So when it comes to the kettle all of that extra energy is being used for no other purpose than to turn the kettle off. It’s not making our water any hotter because it’s already as hot as water can get.
So some questions:
Is there any reason you know of that when making tea or coffee or another hot-style drink we actually need the water to have reached boiling point beforehand? Would water that has reached say 95°C not work or be wrong in some way?
Secondly is there not some way that we could create a mechanism for turning kettles off when the water temperature gets to 95°C? How much might that add to manufacturing costs? Might that cost be acceptable to consumers both on economical and environmental grounds?
Finally has this blatantly already been covered, done, dusted and succeeded or failed, or is on the point of breakthrough, or something in some way that I haven't heard of and therefore I'm either totally behind the times or simply just mildly irrelevant?
I'm obviously worried that perhaps I’ve missed something fundamental here which renders this whole argument pointless, but as it stands there's something tantalisingly promising to my mind about the idea of saving all that energy (which as I’ve found out recently is quite a lot) simply by not having kettles across the world have to be on for as long.
Answers on a postcard (or in the comments) please.